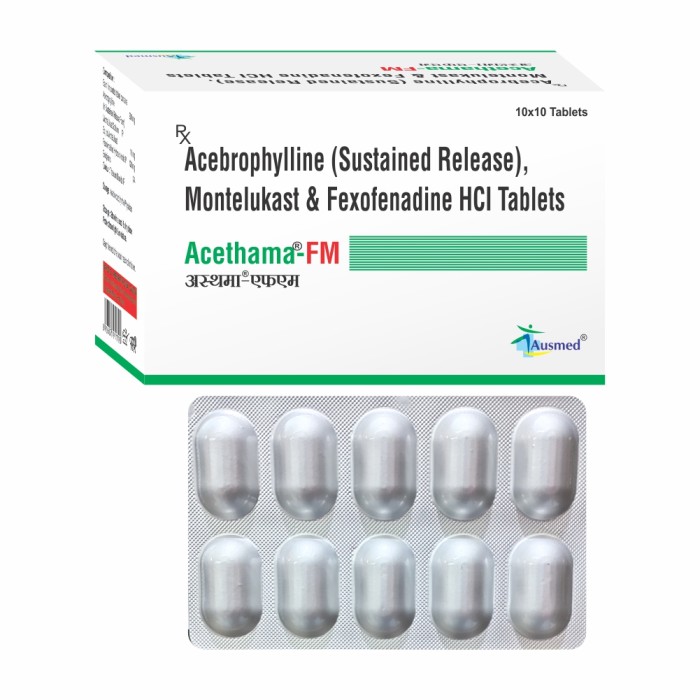
Acebrophylline 200 mg (In Sustained Release Form) Montelukast Sodium IP Eq.to Montelukast 10 mg Fexofenadine Hydrochloride IP 120 mg
215.0 आईएनआर/Strip
उत्पाद विवरण:
- पैकेजिंग (मात्रा प्रति बॉक्स) 10x10 Tablets
- नमक की संरचना Acebrophylline + Montelukast + Fexofenadine
- मेडिसिन की उत्पत्ति India
- दवा का प्रकार
- सामग्रियां Azathioprine (25 mg).
- भौतिक रूप
- खुराक As directed by the physician
- अधिक देखने के लिए क्लिक करें
X
मूल्य और मात्रा
- 100
उत्पाद की विशेषताएं
- Acebrophylline + Montelukast + Fexofenadine
- 10x10 Tablets
- Azathioprine (25 mg).
- India
- As directed by the physician
- Cool and Dry place
उत्पाद वर्णन
Description
- Each Film Coated Tablet Contains Acebrophylline 200 mg (In Sustained Release Form) Montelukast Sodium IP Eq.to Montelukast 10 mg Fexofenadine Hydrochloride IP 120 mg
Doses
- As Directed by physician
Indication
- Fexofenadine is an antiallergic which relieves symptoms like runny nose, watery eyes and sneezing.
- Acebrophylline is a mucolytic which thins and loosens mucus (phlegm), making it easier to cough out. You have been prescribed Montelukast + Fexofenadine + Acebrophylline for prevention of asthma.
FAQs of Acebrophylline 200 mg (In Sustained Release Form) Montelukast Sodium IP Eq.to Montelukast 10 mg Fexofenadine Hydrochloride IP 120 mg:
Q: What is the storage instruction for Acebrophylline 200 mg (In Sustained Release Form) Montelukast Sodium IP Eq.to Montelukast 10 mg Fexofenadine Hydrochloride IP 120 mg tablets?
A: The storage instruction is to keep the tablets in a cool and dry place.Q: What is the packaging quantity per box for Acebrophylline 200 mg (In Sustained Release Form) Montelukast Sodium IP Eq.to Montelukast 10 mg Fexofenadine Hydrochloride IP 120 mg tablets?
A: Each box contains 10x10 tablets.Q: What is the drug type of Acebrophylline 200 mg (In Sustained Release Form) Montelukast Sodium IP Eq.to Montelukast 10 mg Fexofenadine Hydrochloride IP 120 mg tablets?
A: The drug type is general medicines.Q: What is the salt composition of Acebrophylline 200 mg (In Sustained Release Form) Montelukast Sodium IP Eq.to Montelukast 10 mg Fexofenadine Hydrochloride IP 120 mg tablets?
A: The salt composition includes Acebrophylline, Montelukast, and Fexofenadine.Q: What is the dosage recommendation for Acebrophylline 200 mg (In Sustained Release Form) Montelukast Sodium IP Eq.to Montelukast 10 mg Fexofenadine Hydrochloride IP 120 mg tablets?
A: The dosage should be taken as directed by the physician.Tell us about your requirement

Price: Â
Quantity
Select Unit
- 50
- 100
- 200
- 250
- 500
- 1000+
Additional detail
मोबाइल number
Email


 जांच भेजें
जांच भेजें मुझे निःशुल्क कॉल करें
मुझे निःशुल्क कॉल करें
 English
English Spanish
Spanish French
French German
German Italian
Italian Chinese (Simplified)
Chinese (Simplified) Japanese
Japanese Korean
Korean Arabic
Arabic Portuguese
Portuguese