- Home Page
- Company Profile
-
Our Products
- Herbal Products
- Ayurvedic Liver Tablet
- Narica Kesh Oil
- 200 ML Liver Tonic
- 100 ML Anti-Oxidant And L-Lysine With Multivitamin Multimineral Syrup
- 15 ML Multivitamin WIth Zinc Drops
- 100 ML Digestive Enzyme Syrup
- 200 ML L-Lysine With Vitamins And Minerals Syrup
- 300 ML Anti-Oxidant And L-Lysine With Multivitamin Multimineral Syrup
- Red Onion Hair Shampoo
- 225 ML Herbal Enzyme With The Benefits Of Liver Syrup
- 200 ML Tricholine Citrate And Sorbitol Syrup
- RIG VAIDH
- Urology And Nephrology Medicine
- Alfuzosin Hydrochloride IP 10 MG
- Mycophenolate Mofetil IP 500 mg
- Azathioprine IP 50 mg
- Tacrolimus IP 0.5 mg
- Azathioprine IP 25 mg
- Mirabegron extended release tablet 50mg & Solifenacin Succinate IP 5mg
- Tacrolimus IP 2MG
- Solifenacin Succinate 10 mg
- POTASSIUM CHLORIDE IP
- Alpha Ketoanalogues And Essential Amino Acid Tablets
- Sirolimus 1 mg
- Valacyclovir Hydrochloride 1000 mg
- Alfuzosin Hydrochloride Extended Release And DutasterideTablets
- 25 MG Azathioprine Tablets IP
- Azathioprine Tablets IP
- 25 MG Bethanechol Chloride Tablets USP
- 50 MG Bethanechol Chloride Tablets USP
- 100 MG Cyclosporine Capsules IP
- 25 MG Cyclosporine Capsules IP
- 50 MG Cyclosporine Capsules IP
- Nandrolone Decanoate Injection IP
- Nandrolone Decanoate Injection IP
- Nandrolone Decanoate Injection IP
- Alfuzosin Hydrochloride IP 10
- Bethanechol Chloride USP 25 mg
- Bethanechol Chloride USP 50 mg
- Cyclosporine IP 25MG
- Cyclosporine IP 50MG
- Cyclosporine IP 100mg
- Prolonged Release Tablet Contains Darifenacin Hydrobromide IP Eq. to Darifenacin 7.5 mg
- Febuxostat 40 mg
- Finasteride IP 1 mg
- Finasteride IP 5 mg
- Fosfomycin Trometamol oral powder
- Mirabegron extended release tablet 25mg
- Mirabegron extended release 50mg
- Mirabegron 25mg (as extended release Solifenacin Succinate IP 5mg
- Mycophenolate Sodium USP 360 mg
- Nitrofurantoin sustained release 100 mg
- Silodosin 4 mg
- Silodosin 8 mg
- Sodium Bicarbonate IP 1000 mg
- Sodium Bicarbonate IP 500 mg
- Solifenacin Succinate 5 mg
- Tacrolimus IP 1 mg
- Tacrolimus Ointment 0.1% w /w
- Tolterodine tartrate IP 2mg
- Flavoxate HCL IP 200 mg
- Flavoxate HCL 10X15 TABLET
- Valacyclovir Hydrochloride 500 MG
- Nandrolone Decanoate IP 50 mg
- Nandrolone Decanoate IP 25 mg
- Dutasteride IP 0.5 mg
- Silodosin 8 mg Dutasteride IP 0.5 mg
- Erectile Dysfunction Medicine
- Pharmaceutical Injection
- Endocrinology Tablet
- Oncology Medicine
- Tamoxifen CitrateTablets IP
- Lapatinib 250 Mg
- Methotie-2.5 MG Methotrexate Tablets USP
- Methotie 7.5 mg Methotrexate Tablets USP
- Methotie-10 Methotrexate Tablets USP
- Methotie-15 Methotrexate Tablets USP
- 70 MG Dasatininb Tablets
- 250 MG Abiraterone Acetate Tablets IP
- Methotie-5 Methotrexate Tablets USP
- 1 MG Anastrozole Tablets USP
- 50 MG Dasatininb Tablets
- 50 MG Bicalutamide Tablets IP
- 500 MG Capecitabine Tablets IP
- 250 MG Gefitinib Tablets IP
- 500 MG Hydrocyurea Capsules USP
- 400 MG Imatinib Mesylate Tablets IP
- 10 MG Lenalidomide Capsules
- 2.5 MG LetrozoleTablets IP
- 125 MG PalbociclibCapsules
- 100 MG Temozolomide Capsules IP
- 250 MG Temozolomide Capsules IP
- Ophthalmic Medicine
- Nepafenac ophthalmic solution
- Tobramycin & Fluorometholone Ophthalmic solution
- Loteprednol etabonate Ophthalmic solution
- Brimonidine Tartrate Ophthalmic Solution
- Tropicmide eye drop
- Tobramycin & Loteprednol Ophthalmic solution
- Tropicamide & Phenylepherine HCL Eye Drop
- Moxifloxacin Loteprednol Etabonate Ophthalmic suspension
- Polyethylene Glycol 400 +Propylene Glycol IP 0.3% Ophthalmic solution
- 0.25% Alcaftadine Ophthalmic Solution
- 5 ML Atropine Sulphate Ophthalmic Solution 0.01% W-V USP
- 1.5% W-V Bepotastine Besilate Ophthalmic Solution
- 3 ML Bimatoprost Ophthalmic Solution 0.03% W-V Sterile Eye Drops
- Brimonidine Tartrate And Tomolol Maleate Ophthalmic Solution
- Brimonidine Tartrate Ophthalmic Solution IP
- 1% W-V BrinzolamideOphthalmic Suspension IP
- 0.09% W-V Bromfenac Ophthalmic Solution
- Potassium Iodide Sodium Chloride And Calcium Chloride Eye Drops
- 5 ML Clotrimazole And Lignocaine Hydrochloride Ear Drops
- 5 ML Cyclopentolate Ophthalmic Solution IP 1% W-V Eye Drops
- Dorzolamide HCl With Timolo Maleate Ophthalmic Solution IP
- 5 ML Dorzolamide Eye Drops 2% IP
- 10 ML Naphazoline Phenylephrine CPM Menthol And Camphot Eye Drops
- Fluorometholone Ophthalmic Suspension IP
- Flubiprofen Sodium Ophthalmic Solution IP
- GatifloxacinAnd Flurbiorifen Sodium Eye Drops
- 5 ML GatifloxacinAnd Prednisolone Acetate Eye Drops
- GatifloxacinOphthalmic Solution
- Alcaftadine 2.5 mg
- Ciprofloxacin + HPMC Ophthalmic Solution
- Ciprofloxacin Dexamethasone eye drop
- Atropine Sulphate Opthalmic Solution
- Bepotastine Besilate Ophthalmic solution
- Bimatoprost Ophthalmic Solution 0.03%w/v
- Brimonidine Tartrate Timolol Maleate Ophthalmic solution
- Brimonidine And Brimonidine Tartrate Ophthalmic solution
- Bromfenac ophthalmic solution 0.09% w/v
- Potassium Iodide IP 3.3% w/v Sodium Chloride IP 0.83% w/v Calcium Chloride IP 1% w/v Chlorbutol IP 0.5% w/v
- Clotrimazole + Lignocaine HCI +Propylene Glycol Ear Drops
- Cyclopentolate HCI I.P. 1% w/v + Chlorbutol I.P. 0.5% w/v, Ophthalmic solution
- Cyclosporine 0.05% w/v ophthalmic solution
- Dorzolamide 2% w/v ophthalmic solution
- Dorzolamide HCL 2% w/v + Timolol Maleate 0.5% w/v Ophthalmic Solution
- Gentamicin IP 0.3% w/v + Beclomethasone Dipropionate IP 0.025% w/v +Clotrimazole 1%+Lignocaine Hydrochloride IP 2% w/v. % w/v +
- Naphazoline , Phenylephrine, CPM Menthol & Comphor Eye Drop
- Fluconazole IP 0.3% w/v
- Fluorometholone 0.1% w/v 5 ml
- lurbiprofen sodium 10ml Ophthalmic solution
- Flubiprofen 0.03% +Hydroxypropylmethylcellulose 0.25% 5Ml Solution
- Homatropine Hydrobromide IP 2% + eye drop
- Sodium Hyaluronate B.P. 0.1% 10ML Solution
- Ketorolac Tromethamine IP 0.5% Eye drop
- Ketorolac Tromethamine IP 0.5% + Phenylephrine HCL IP 0.012% + CPM 0.02% 5ML
- Ketorolac + Ofloxacin Ophthalmic solution
- Latanoprost IP 0.005% + Ophthalmic solution
- Latanoprost + Timolol Maleate Ophthalmic solution
- Levofloxacin + Ophthalmic solution
- Sodium Hyaluronate + D-Panthenol + Ophthalmic solution
- Moxifloxacin Ophthalmic solution
- Moxifloxacin Dexamethasone Ophthalmic solution
- Moxifloxacin + Difluprednate Ophthalmic solution
- Moxifloxacin Ketorolac Tromethamine
- Moxifloxacin Hydrochloride +paraffin Oinment
- Moxifloxacin & Prednisolone Ophthalmic suspension
- Moxifloxacin & Tobramycin
- Natamycin Ophthalmic suspesion
- Olopatadine Hydrochloride 0.1% w/v Ophthalmic solution
- Ofloxacin C Dexamethasone Sodium Phosphate
- Ofloxacin Ophthalmic solution
- Polyvinyl Alcohol with Povidone Eye drop
- Prednisolone Acetate IP 1% w/v ophthalmic solution
- Hydroxypropyl Methyl cellulose Ophthalmic Solution
- Travoprost & Timolol maleate Eye drop
- Sodium Carboxymethly- Cellulose Ophthalmic solution
- Sodium Carboxymethly- Cellulose 1% w/v ophthalmic solution
- Timolol Maleate 0.5% w/v eye drop
- Tobramycin Sulphate Ophthalmic solution
- Tobramycin Sulphate Dexamethasone Sodium Phosphate HPMC
- Travoprost Ophthalmic solution
- Brinzolamide ophthalmic solution
- Chloramphenicol Eye Drop
- Gatifloxacin Ophthalmic solution
- Gatifloxacin & Flurbiprofen Sodium eye drop
- Gatifloxacin & Prednisolone Acetate eye Drop
- Chloramphenicol With Polymyxin B Sulphate
- Paradichlorobenzene , Benzocaine,Chlorbutol, Turpentine OIL ear drop
- Dicyclomine HCL IP 10 mg + Chlorbutol IP 0.5% w/v (as preservative) Water for injection
- Diacerein IP 50mg.
- ABOYVIR EYE OINTMENT
- Gynecologic Medicine
- Clomish-25 Clomifene Tablets IP
- Clomish-50 Clomifene Tablets IP
- Vitamin-D3 Myo-Inositol And D-Chiro-Inositol Sachet
- 2.5 MG Bromocriptine Mesylate Tablets IP
- 0.25 MG Cabergoline Tablets IP
- 0.5 MG Cabergoline Tablets IP
- 1 MG Cabergoline Tablets IP
- Clindamycin And Clotrimazole Vaginl Soft Gelatin Capsules
- 100 MG Danazol Capsules IP
- 200 MG Danazol Capsules IP
- Cyproterone Acetate And Ethinylestradiol Tablets
- Doxylamine Succinate And Pyridoxine Hydrochloride And Folic Acid Tablets
- 2 MG Estradiol ValerateTablets
- 10 MG Medroxyprogesterone Acetate Tablets IP
- Natural Micronised Progesterone Soft Gelatin Capsules
- Natural Micronised Progesterone Soft Gelatin Capsules
- Progesterone Soft Gelatin Capsules
- Progesterone Sustained Release Tablets
- Noradnalin Bitartrate Injection IP
- Norethisterone Tablets IP
- Myo-Inositol, D-Chiro-Insositol, L-methyfolate Multivitamins & Anti-Oxidants Softgelatin Capsule
- Bromocriptine Mesylate 2.5mg
- Cabergoline IP 0.25 mg
- Cabergoline IP 0.5 mg
- Cabergoline IP 1 mg
- CARE VAGINAL WASH
- Clindamycin & Clotrimazole Vaginal soft Gelatin Capsules
- Danazol IP 100mg
- Danazol IP 200mg
- Cyproterone Acetate & Ethinylestradiol
- Doxylamine succinate & Pyridoxine hydrochloride & Folic Acid
- Dydrogesterone IP
- Clomiphene Citrate IP 25 mg
- Clomiphene Citrate IP 50 mg
- Dydrogesterone IP 10 mg,
- Estradiol Valerate 2mg tablet
- Calcitriol Omega 3 Fatty Acids (EPA -DHA) Methylcobalamin folic Acid , Calcium carbonate soft gelton capsule
- Medroxyprogesterone Acetate IP 10 mg,
- Natural Micronised Progesterone
- Progesterone Sustained Release Tablet
- Progesterone IP 300 mg.
- Norethisterone 5mg
- Natural Micronised Progesterone soft gelton capsules
- Noradrenaline Bitartrate IP 2mg injection
- Myo-inositol ,D-Chiro-inositol , Vitamin D3 Sachet
- L-Arginine 3.00g + Grape Seed Extract Sachet
- Hydroxyprogesterone Caproate Injection
- Hydroxyprogesterone Caproate IP 250 mg Injection
- Evening Primose Oil soft gelatin capsule
- Testosterone Enanthate Injection
- Testosterone Enanthate Injection
- Tranexamic Acid IP 500 mg
- Tranexamic Acid Injection
- Tranexamic Acid & Mefenamic Acid
- Dicyclomine HCL & paracetamol suspension
- Clotrimazole IP 100mg TABLET
- Pain Relief Medicine
- Body Zox-Fort Diclofenac Potassium Paracetamol And Chlozoxazone Tablets
- Body Zox-MR Diclofenac Potassium Paracetamol And Chlozoxazone Tablets
- Iburofen And Paracetamol Tablets IP
- 60 ML Mefenamic Acid And Paracetamol Suspension
- 60 ML Dicyclomine Hydrochloride And Paracetamol Suspension
- Trypsin And Chymptrypsin Tablet
- Trypsin Bromelain Trihydrate And Diclofenac Sodium Tablets
- Flupirtine Maleate And Paracetamol Tablets
- Paracetamol Phenylephrine Hydrchloride And Chlorpheniramine Maleate Tablets
- Drotaverine And Mefenamic Acid Tablets
- 50 MG TapentadolCapsules
- Diclofenac Potassium ,Paracetamol , Chlorzoxazone
- Diclofenac Potassium ,Paracetamol, & Chlorzoxazone
- Paracetamol ,Phenylephrine Hydrochloride,Chlorpheniramine maleate
- Ibuprofen & Paracetamol
- Mefenamic Acid & Paracetamol suspension
- Mefenamic Acid & Dicyclomine hydrochloride
- Caroverine Hydrochloride 20mg
- Trypsin & Chymotrpsin tablet
- Trypsin , Bromelain , Rutoside Trihydrate & Diclofenac Sodium
- Aceclofenac ,Paracetamol,trypsin & chymotrypsin
- Drotaverine Hydrochloride & Mefenamic
- FLUPIRTINE MALEATE & PARACETAMOL
- Tolperisone hydrochloride 150mg.
- Tapentadol 50mg
- Hyoscine Butylbromide IP 10 mg Mefanamic acid IP 250 mg
- Hyoscine Butylbromide IP 10mg
- HIV And Aids Medicine
- Lopinavir And Ritonavir Tablets IP
- 600 MG Efavirenz Tablets IP
- Tenofovir Disoproxil Fumarate Lamivudine And Efavirenz Tablets
- 300 MG Tenofovir Disoproxil Fumarate Tablets IP
- Lopinavir & Ritonavir tablet
- Efavirenz 600MG
- Tenofovir Disoproxil Fumarate,Lamivudine , Efavirenz
- Tenofovir Disoproxil Fumarate 300MG
- Tenofovir Alafenamide 25mg
- General Medicine
- 100 ML Disodium Hydrogen Citrate Syrup
- 10 MG AtomoxetineHydrochloride Tablets
- 100 ML Levodropropizine And Chlorpheniramine Maleate Syrup
- Injection Of Vitamin B-Complex With Vitamin B12
- Vitamin B-Complex With L-Lysine Capsule
- 10 MG Biotin Tablets
- 5 MG Biotin Tablets
- MultiMinerals, Ginseng & Cyanocobalamin Softgel Capsule
- Antioxidant, Multivitamin, Multiminerals and Methylcobalamin Tablets
- ayurvedic cracked heel repair cream
- Disodium Hydrogen Citrate Syrup
- Disodium Hydrogen Citrate Syrup
- Ambroxol Hydrochloride ,Levocetirizine Dihydrochloride & Menthol Syrup
- Levosulbutamol Sulphate ,Ambroxol Hydrochloride, &Guaiphenesin
- Atom oxetine Hydrochlorid
- Levodropropizine ,Chlorpheniramine,Maleate Syrup
- Vitamin B-complex with L-Lysine Capsules
- Injection of vitamins B with Vitamin B12
- Injection of vitamin B complex With Vitamin 12
- Vitamin B complex With L-Lysine Syrup
- Vitamin B -Complex with L-Lysine Hcl Syrup
- Biotin IP 10mg
- Biotin IP 5mg
- Multivitamin, Multimineral & Antioxidant Softgel Capsules
- Calcium carbonate & vitamin D3 Suspension
- Vitamin B-complex Capsule
- Dextro methorphan Hydrobromide,Phenylephrine Hydrochloride ,Paracetamol suspension
- Paracetamol ,Chlorpheniramine Maleate& Phenylephrine Hydroride
- Cholecalciferol I.P 60,000 IU SOFT GELTAN CAPSULE
- Cholecalciferol , (Vitamin D3 ) Oral Drop
- Cholecalciferol Injection
- Vitamin D3 Oral Solution 60000 IU
- Vitamin D3 oral spray
- Cholecalciferol,Vitamin D3 Oral Solution
- Cholecalciferol sachet
- Melatonin 0.5 mg oral spray
- Multivitamin Infusion Injection
- Oral Rehydration Salt
- Omega-3 Fatty Acid,Coenzyme Q10,Lyhcopien Selenium,Wheat Germ oil , L-Arginine, L-Carnitine , & Zinc Soft Geltan Capsule
- Diclofenac Potassium,Paracetamol Cetirizine Dihydrochloride & Magnesium Trisilicate
- Zinc Sulphate Dispersible tablet
- Zinc Gluconate suspension
- Zinc Gluconate suspension 60ml
- Terbutaline Sulphate ,Bromehexine Hydrochloride, Guaiphenesin, Menthol syrup
- Ambroxol Hydrochloride,Terbutaline sulphate ,Guaiphenesin, Menthol
- Terbutaline Sulphate, Bromhexine Hydrochloride, Guaiphenesin, Menthol Syrup
- Terbutaline Sulphate, Bromhexine Hydrochloride, Guaiphenesin, Menthol Syrup
- Terbutaline Sulphate, Bromhexine Hydrochloride, Guaiphenesin, Menthol Syrup
- Clindamycin Phosphate Injection
- Clindamycin phosphate 1% GEL
- Cetirizine Hydrochloride Tablet
- Paracetamol, PhenylephrineHydrochloride,Caffeine, Diphenhydramine Hydrochloride
- Cetirizine HCI 10MG
- Paracetamol, Cetirizine HCL,& Chlorpheniramine Maleate
- Each ml contains: Paracetamol I.P 125MG + Cetirizine HCL I.P 2.5MG + Phynlephrine HCL I.P 2.5MG DROP
- Paracetamol,Phenylephrine,Hydrochloride,Chlorpheniramine Suspension
- Paracetamol,Cetrizine Hydrochloride,Chlorpheniramine Maleate Suspension
- Cetirizine Hydrochloride suspension
- Pyridoxine Hydrochloride ,Niacinamide ,Cyanocobalamin,Folic Acid with Chromium Zinc & Selenium tablet
- Metronidazole,Loperamide Hydrochloride & Dicyclomine Hydrochloride tablet
- Mecobalamin,Alpha Lipoic Acid, Inositol , Benfotiamine , Folic Acid,Chromium Polynicotinate,& Selenium Dioxide Monohydrate soft geltan capsule
- Vitamin E,Wheat Germ Oil ,Omega-3 Fatty Acid soft geltan capsule
- Vitamin E, Wheat Germ Oil & Omega-3 Fatty Acid Soft gelatin Capsules
- Tocotrienols,Resveratrol , Lutein ,Astaxanthin & Zeaxanthin Soft geltan capsule
- Ferric Ammonium Citrate,Cynocobalamin,Folic Acid syrup
- Iron Vitamin B12,& Folic Acid Drop
- Ferrous Ascorbate & Folic Acid Tablet
- Carbonyl Iron,Folic Acid ,& Zinc Sulphate Monohydrate Capsule
- Shudh Shilajeet,Swait Parpati,Moolishar ,Sheetal chini ,Saindha Namak , Sajjikhar, Punarnava, Panchtrin Mool, Ikshu Mool, Gokhru, Varun Twak , Kulattha, Pashan Bhed, Palashpushp, Makoi , Kakri Beej, Daruharidra syrup
- Ayurvedic Stone tonic
- Ayurvedic Stone Tablet
- Folic Acid Tablet
- Paracetamol ,Phenylephrin HCL & Levocetrizine Dihydrochloride suspension
- Calcitriol , Calcium Carbonate,Zinc Sulphate Monohydrate soft geltan capsule
- Calcitriol,Calcium Carbonate, Zinc Sulphate Monohydrate soft gelton capsule
- Omega 3 Fatty Acid, Cyanocobalamin, Calcitriol, Calcium & Folic Acid soft gelton capsule
- Iron (III) Hydroxide Polymaltose complex equivalent to elemental iron 50MG & folic acid 1MG suspension
- ferric Ammonium Citrate,Iron,Folic Acid,Cyanocobalamin SYRUP
- Ibuprofen IP 100 mg. Paracetamol IP 125mg Suspension'
- Paracetamol I.P 325 MG Bromhexine HCL IP 4mg Guiphenesin IP 50 mg Phenylepherine Hydrochloride I.P 5 Chlorpheniramine maleate IP 2mg tablet
- Chlorpheniramine Maleate IP 2 mg. Dextrom ethorphan Hydrochloride IP 10 mg. Phenylephrine Hdyrochloride IP 5 mg tablet
- DiPhenhydramine HCL I.P. 25MG soft gels
- Dipphenhydramine Hydrochloride I.P. 14.08 MG=Ammonium Chloride I.P 138 Ammonium Chloride I.P. 57.03MG=Sodium citrate I.P. 1.14 mg syrup
- Folic Acid IP 5 mg tablet
- Levosalbutamot Tartrate 100mcg + Ipratopium Bromide IP 40mcg respkaps
- Levocarnitine IP 500 mg tablet
- Camphor USP 25 mg Chlorothymol 5 mg Eucalyptol USP 125 mg Menthol IP 55 mg Terpineol BP 120 mg Soft gelatin capsule
- Lycopene, GENERAL & Multimineral Syrup
- Antioxidant, Multivitamin, Multiminerals & Lycopene capsule
- Lycopen with multiminerals & multivitamins soft geltan capsule
- Calcium Citr ate ,Vitamin D3 , Minerals tablet
- Methylcobalamin IP 2500 mcg Injection Disposable Syringe With a Needle.
- Methylcobalamin IP 500 mcg Tablet
- Methylcobalamin, Benzyl Alcohol Injection 1 ml
- Methylcobalamin IP 1500 mcg injection
- Methylcobalamin IP 1500 mcg. + Benzyl Alcohol IP 2% v/v + Water for Injection
- Cyanocobalamin 1000 MCG. Pyridoxine Hydrochloride IP 100 MG. Nicotinamide IP 100 MG. D-Panthenol 50 MG, Injection
- Cyanocobalamin IP 1000 mcg. Pyridoxine HCl IP 100 mg. Niacinamide IP 100 mg. D-Panthenol IP 50 mg. Benzyl Alcohol IP 2% v/v Water for Injection
- L-Carnitine L-Tartrate Eq.to L-Carnitine 500mg +Methylcobalamin IP 1500mcg +folic Acid IP 1.5mg Tablet
- Minoxidil IP 10%w/v (Alcohol Free)
- Minoxidil IP 5%w/w In a Alcohol free
- Minoxidil IP 5mg tablet
- Minoxidil IP 5%w/v + Finasteride IP 0.1%w/v + Soluble base spray
- Montelukast SodiumIP eq. to Montelukast 10 mg. + Levocetirizine HCI IP 5 mg tablet
- Levocetirizine Dihydrochloride IP 2.5 mg. Montelukast Sodium IP 4 mg. suspension
- Montelukast SodiumIP 10 mg. Levocetirizine HCI IP 5 mg tablet
- methylcobalamin 1500mcg + Alpha Lipoic Acid 200 MCG + Folic Acid 1.5MG+ Pyridoxine Hydrochloride I.P 3 MG + Biotin BP 30 mcg capsule
- Nimesulide BP 100mg tablet
- Nimesulide BP 100 mg. Phenylephrine Hydrochloride IP 5 mg Levocetirizine Hydrochloride IP 5 mg tablet
- Nimesulide BP 100 mg. Paracetamol IP 325 Phenylephrine Hydrochloride IP 10 mg Caffeine ,Anhydrous, IP 25 mg tablet,
- Nimesulide BP 100 mg Paracetamol IP 325 mg tablet
- Nimesulide BP 100 mg, Paracetamol 325 mg tablet
- Nimusulide BP 100 mg. tablet
- Nutritional Info : Each Sachet 7g powder contains Approx ( Energy 6.24 kcal + Carbohydrate 1.56 g + Protein 0.00g + Fat 0.00g + L-Glutamine 5.00g ) sachet
- Omega-3 Omega 6 Omega 9 essential fatty Acid, Derived form Flax Seed oil 500 mg (Alpha Linolenic Acid 253 mg, Linoleic acid 88.3 mg, Oleic acid 21.3 mg) Capsule
- Omegro-3 Fatty Acid 150mg , Ginseng Extract 42.5mg ,Wheat Germ oil 25mg, Citrus Bioflavonoids (8mg Bioflavonoids) 20mg, Grape Seed Extract 15mg, Green Tea Extract 10mg, capsule
- OMEGRO -5G
- OMEGRO-12G SOFTGEL CAPSULE
- OMEGRO -9G
- Orl istat USP 120 mg Capsule
- Antioxidant, Lycopene, GENERAL, Minerals, Ginseng & Cyanocobalamin Softgel.
- Multivitamin, multimineral , Antioxidat soft geltan capsule
- Paracetamol IP 1.0% 100 ml Injection
- Paracetamol I.P. 300 mg (As Immediate Release) Paracetamol I.P. 700 mg (As Sustained Release) tablet
- Paracetamol IP 500 mg tablet
- Paracetamol I.P 650 MG tablet
- Paracetamol Ip 250 mg oral suspension
- Paracetamol IP 125 mg, (Flavoured syrupy base )
- Paracetamol IP 75 mg Benzyl alcohol (as preservative) IP 1.5% v/v Propylene Glycol IP , Water for injection IP
- Povidone lodine,Metronidazole ointment
- Povidone-lodine Oinment
- Povidone-lodine oinment
- Povidone-lodine I.P 10% w/w (Available lodine 10%w/v) water soluble ointmnt
- Povidone-Iodine IP 5%, Sucralfate U.S.P. 7% Oinment
- Protein Powder With Vitamins & Minerals (nutritional supplement) VANILA FLAVOUR
- Protein Powder With Vitamins & Minerals (nutritional supplement) CHOC. FLAV
- Protein Powder With Vitamins & Minerals (nutritional supplement) KESAR BADAM
- Racecadotril IP 10mg sachet
- Racecadotril IP 100mg Hard gelatin capsule
- Methylcobalamin IP 1500 mcg, Alpha Lipoic acid IP 100mg , Benfotiamine 150mg, Inositol IP 100mg , Chromium Picolinate IP 200mcg, Pyridoxine HCL IP 3mg, Folic Acid IP 1.5mg, Vitamin D3 IP 1000 IU, Calcium Carbonate IP 500mg
- Methylcobalamin IP 1500 mcg Alpha Lipoic acid usp 200 mg Folic acid IP 1.5 mg Chromium Picolinate USP 200 mcg Seleno Methionine USP 55 mcg Inositol usp 100 mg zinc Monomethionine BP 25 mg soft gelatin capsule
- Chlorhexidine Gluconate Solution IP 0.3% v/v Cetrimide IP 0.6% w/v ANTISEPTIC LIQUID 1 LTR
- Chlorhexidine Gluconate Solution IP 0.3% v/v Cetrimide IP 0.6% w/v ANTISEPTIC LIQUID 100 ML
- Chlorhexidine Glucconate Solution IP Diluted to Chlorhexidine Gluconate 0.20% w/v + Sodium Fluoride IP 0.05%w/v + Zinc Chloride IP 0.09% w/v In pleasantly flavour Aqueous base q.s. Mouth wash
- Chlorhexidine Gluconate Solution IP Diluted to Chlorhexidine Gluconate 0.2%w/v Mouth Wash
- Chlorhexidine Gluconate Solution IP Diluted to Chlorhexidine Gluconate 0.2%w/v Mouth wash 450 ml
- Potassium Nitrate & Sodium Monofluorophosphate Gel
- Ferric Hydroxide Complex with Suckrose,Elemental Iron 20 mg Injection
- Ferric Carboxymaltose equivalent to elemental Iron 50mg. Water for injection
- Thiamine Hydrochloride IP 100mg Tablet
- Thiamine Hydrochloride IP 100mg tablet
- Thiamine Hydrochloride IP 100mg Chlorbutol IP 0.35% w/v (as preservative) Water for injection
- Tocotrienol Soft Geletin capsule
- Dextr o methorphan Hydrobromide 10 MG +Ambroxol Hydrochloride IP 15mg + Chlorpheniramine Maleate IP 2 mg + Phenylephrine Hydrochloride IP 5mg , Menthol IP 1.5 mg Suspension
- Multivitamin, Multimineral & Antioxidant Softgel Capsules
- Digestive Enzyme B.Complex With Lysine Suspension
- Fungal Diastase I.P. (1 1200) (Fungal Diastase derived from Aspergillus oryzae digests not less than 60 gm of cooked starch) Pepsin I.P. (1 3000) 10 mg (Digests not less than of coagulated egg albumen) Flavoured syrup base
- Energy 8.88kcal + Carbohydrate 0.22g + Sugar 0.15g. + Protein 0.00g. + Fat 0.00g. + L-Lysine Hydrochloride 3.33mg + Diastase 1.33mg + Papain 0.67mg + Caraway 26.67mcg. + Cardamom 26.67 mcg. + Cinnamon 1.33mcg DROP
- Anti Infective Medicine
- Amytum-500 Cefuroxime Axetil Tablets IP
- 400 MG Aciclovir Tablets IP
- 800 MG Aciclovir Tablets IP
- Albendazole Tablets IP
- 10 ML Ivermectin And Albendazole Suspesion
- Albendazole And Ivermectin Tablets
- 500 MG Amikacin Sulphate Injection IP
- Cefuroxime Axetil Tablets IP
- Cefuroxime Axetil And Potassium Clavulanate Tablets
- Artemether And Lumefantrine Tablets IP
- Aciclovir IP 800 mg tablet
- Albendazole IP 400 mg tablet
- Albendazole IP 400 mg. + Ivermectin 6 mg tablet
- Ivermectin IP 3 mg. Albendazole IP 200 mg. Suspension
- Albendazole oral suspension I.P.200MG
- Amikacin Sulphate Injection I.P 500MG
- Cefuroxime Axetil 250mg TABLET
- Cefuroxime Axetil 500MG Tablet
- Arteether (A-B Arteether) IP 150 mg Injection
- Cefuroxime Axetil 500mg ,Potassium Clavulanate 125mg. TABLET
- Artemether IP 80 mg,Lumefantrine 480 mg tablet
- Amoxycillin 500 mg. + Potassium Clavulanate Diluted IP Equivalent to Clavulanic Acid 125 mg TABLET
- Amoxycillin 875 mg Potassium Clavulanate Diluted Clavulanic Acid 125 mg TABLET
- Amoxycillin Trihydrate IP equivalent to Amoxycillin 250 mg Potassium Clavulanate Diluted IP Equivalent to Clavulanic Acid 125 mg TABLET
- Amoxycillin Trihydrate IP Equivalent to Amoxycillin 500 mg. Potassium Clavulanate Diluted IP Equivalent to Clavulanic Acid 125 mg Tablet
- Amoxycillin Trihydrate I.P 200 mg eq. to Amoxycillin, + Potassium Clavulanate Diluted I.P. 28.5mg eq. to Clavulanic Acid, Excipients q.s.(in a flavoured base) suspension
- Amoxycillin Trihydrate IP eq. to Amoxycillin Anhydrous 400 mg Potassium clavulanate Diluted IP eq. to Clavulanic acid 57 mg, Suspension
- Amoxycilline Sodium Potassium Clavulanic, Injection
- Amoxycillin,Potassium clavulante, Lactic Acid Bacillus tablet
- Artesunate Injection
- Artesunate Injection i.p 120mg
- Azithromycin oral suspension 100mg.
- Azithromycin IP 200 mg Oral Suspension
- Azithromycin Anhydrous 250mg tablet
- Azithromycin Anhydrous 500mg tablet
- Azithromycin-500 mg Injection
- Amoxycillin (anhydrous) 500mg tablet
- Clotrimazole ,Beclomethasone Diprop creamionate
- Indapamide sustained release 1.5 tablet
- Clindamycin Hydrochloride IP 150 mg tablet
- Clindamycin Hydrochloride IP 300 mg tablet
- Ceftazidime IP (Sterile) 250 mg Injection
- Cefotaxime Sodium IP (Sterile) Anhydrous Cefotaxine 1000 mg Doses Injection
- Clarithomycin IP 125mg supension
- Clarithromycin IP 500 mg tablet
- Clotrimazole , Glycerine , Propylene Glycol MOUTH PAINT
- Colistin Sulphate oral suspension
- Colistimethate sodium Injection
- Colistimethate sodium Injection 2MIU
- Colistimethate sodium Injection 3MIU
- Daclatasvir 60mg Tablet
- Doxycycline 100 mg Injection
- Doxycycline 100 mg + Lactic Acid Bacillus capsule
- Doxycycline 100mg Lactic acid Bacillus capsule
- Doxycycline 100 mg + Lactic Acid Bacillus capsule
- Faropenem 200 mg tablet
- Faropenem 300 mg Extended Release Tablet
- Faropenem sodium ,potassium clavulanate, Tablet
- Fluconazole IP 150 mg Tablet
- Fluconazole I.P 150MG Tablet
- Fluconazole IP 200mg tablet
- Fluconazole IP 400mg tablet
- Combikit Fluconazole, Azithromycin, Secnidazole Tablets
- Cefixime IP 100 mg. Tablet
- Cefixime IP 200 mg tablet
- Cefixime (As Trihydrate IP) eq. to anhydrous Cefixime 200 mg tablet
- Cefixime oral suspension 10ml
- Cefixime oral suspension 30ml
- Cefixime IP 200 mg. Ofloxacin IP 200 mg. TABLET
- Cefixime 50 mg. + Ofloxacin IP 50 mg. WITH SPOON SUSPENSION
- Gentamicin INJECTION 2ML
- Gentamicin INJECTION 30ML
- Imipenem & Cilastatin Sodium INJECTION
- Itraconazole BP 100 mg CAPSULE
- Itraconazole BP 200 mg TABLET
- Itraconazole BP 50 mg Capsule To Treat Skin Fungal Infections
- Itraconazole BP 50 mg capsule
- Ivermectin IP 12 mg tablet
- Ivermectin USP 6 mg tablet
- Ivermectin 1% w/w Cream
- Ketoconazole I.P. 200 mg tablet
- Lamotrigine IP 100mg tablet
- Lamotrigine IP 50mg tablet
- Levofloxacin 500 mg tablet
- Linezolid IP 600 mg tablet
- linezolid oral suspension 30ml
- Linezolid Injection 300ml
- Cefoperazone Sulbactum Injection
- Sulbactum Sodium Sulbactum Injection
- Cefoperazone & Sulbactum injection
- Propylene Glycol IP 4.96% w/w + Carbomer IP 0.76% w/w + Colloidar Silver 32 ppm + Triethanolamine BP 0.32% w/w + Purified Water IP q.s. gel
- Meropenam Injection
- Meropenem I.P injection 500mg
- Meropenem Sulbactam Injection
- Metronidazole IP 400 mg tablet
- Metronidazole Extended Release 600mg tablet
- Metronidazole 1.5% w/w GEL
- Miconazole Nitrate IP 2.00%, Cream
- Miconazole Nitrate IP 2.00%, Cream
- Ceftriaxone INJECTION
- Ceftrixone Sodium IP Injeciton IP 250 mg
- Ceftrixone Sodium IP Injeciton IP 500 mg
- Ceftrixone Sodium IP 1000 MG. Sulbactum Sodium USP 500 mg injection
- Ceftriaxone Sodium I.P. (Sterile) eq. to Anhydrous Ceftriaxone 250MG Sulbactam Sodium 125 MG & Sterile Water for injection I.P 5 ml. elsewhere and packed with in this combi - pack Injection
- Ceftriaxone 1000 mg Sterile Tazobactam Sodium Eqv to Tazobactam 125 mg injection
- Cefpodoxime Proxetil Potassium Clavulanate Tablet
- Cefpodoxime Proxetil & Potassium Clavulanate Oral Suspension
- Cefpodoxime Proxetil I.P. eq. to Cefpodoxime 100 mg (Anhydrous) Potassium Clavulanate Diluted IP eq. to Clavulanic acid 62.5mg oral suspension
- Cefpodoxime Proxeil IP 50 mg. Potassium Clavunanate Diluted IP 31.25 mg. Dry syrup
- Cefpodoxime Proxetil I.P. eq. to Cefpodoxime 100 mg Oral suspension
- Cefpodoxime Proxetil IP 25 mg oral suspension
- Cefpodoxime, Oral suspension 30ml
- Cefpodoxime Proxetil Oral suspension 30ml
- Cefpodoxime Proxetil Dispersible 100 mg. tablet
- Cefpodoxime 200 mg tablet
- cefpodoxime 200MG + Ofloxacin I.P 200MG tablet
- Moxifloxacin Hydrochloride BP 400 mg tablet
- Norfloxacin IP 400mg Tablet
- Norfloxacin IP 400mg +Tinidazole IP 600mg tablets
- Ofloxacin IP 200 mg Tablet
- Ofloxacin IP 200 mg. tab ( Blue )
- Ofloxacin IP 200 mg. tab ( Red )
- Ofloxacin IP 50 mg + Ornidazole IP 125 mg + Racecadotril IP 15 mg SYRUP 30ML
- Ofloxacin IP 200 mg. Ornidazole IP 500 mg TAB
- Ofloxacin IP 50 mg oral suspension 60ml
- Ofloxacin IP 400 mg tab
- Povidone-lodine Solution IP 5% 100ml
- Povidone-lodine Solution 500ml
- Povidine -Iodine Gargle 100ml
- Povidone-lodine Solution 10% 100ml
- Povidone-lodine Solution IP 10% 500ml
- Povidone iodine IP 5% POWDER
- Povidine Iodine 7.5% Cleansing SCRUB
- Ribavirin IP 200mg CAP
- Permethrin 5% LOTION 50ML
- Permethrin 5% Ointment
- Permithrin BP 1% w/w + Glycerin IP 2% w/w Soap
- Cephalexin 500MG CAP
- Sultamicillin Tosilate Dihydrate B.P. 375 mg TAB
- Piperacillin 4g + Tazobactum 0.5g Injection
- Teicoplanin IP 400 mg injection
- Terbinafine 250 mg, Terbicare tablet
- Terbinafine Hydrochloride 500 mg tab
- Terbinafine hydrochloride IP 1% Cream
- Tigecycline Powder For Injection 50mg
- Choline Salicylate ,Lignocaine Hydrochloride Gel
- Vancomycin HCI Injection
- Vancomycin HCI Injection
- Vitamin C L-Glutathion Hyaluaric Acid Facial Serum
- Cosmetic Products
- Narica Hair Removal Cream
- 30 ML Narica Facial Serum
- Narica Cold Cream
- Coffee Face Wash
- 100 ML Activated Charcoal Face Wash
- Narica Body Wash
- Apple CiderVinegar Foaming Face Wash
- Skincare Glycerin
- Haldi Chandan Face Wash
- Kiwi Face Wash
- Aloevera And Vitamin E, moisturising Lotion
- Natural Breast Firming Cream
- Natural Breast Firming Oil 100ml
- Natural Breast Firming Cream
- Skin Care Glycerin
- GLYCERIN 1L.
- GLYCERIN LOTION
- Apple Cider vinegar, Aloe vera Extract, Vitamin B5 & E, FACE WASH
- Soapwort Extrat Rice Extract With Coconut Oil BODY WASH 200ml
- Enrich with Active Charcoal & Vitamin E CHARCOAL FACE WASH
- Anti Dullness & skin brightning COFFEE FACE WASH
- Cucumber Extract Rose Extract Aloevera Extract COLD CREAM
- Aloevera and Vitamin E, Hair Removal cream
- Enriched with Haldi Chandan extract face wash
- Enriched with Aloevera and Kiwi Extract face wash
- Lip Balm (Protect Dryness, Chapping & Cracking Lips
- Moisturing Lotion With Vitamin E
- Enriched with Papaya Face Wash
- Purified water, Propellant Gas, Denatured Ethyl Alcohol (95% v/v), Propylene Glycol, Cyclopentasiloxane & Fragrance. BODY SPRAY
- Petroleium Jelly 99.00% + coconut oil (Coco Nucifera ) 0.50% & Olive oil ( Olea europaea)* 0.50%
- SUN SCREEN LOTION 30 SPF
- SUN SCREEN LOTION 50 SPF
- Petroleium Jelly
- SUNSCREEN SPRAY SPF 70 ( FOR ALL SKIN TYPE)
- Tea Tree face wash
- Vitamin-C face wash
- Vitamin C serum 30ML
- Anti Diabetic Medicine
- Glicaday-M 80 Gliclazide And Metformin Hydrochloride Tablets
- Glicaday-80 MG Gliclazide Tablets IP
- 5 MG Dapagliflozin Tablets
- Dapagliflozin And Teneligliptin Tablets
- 50 MG Epalrestat Tablets IP
- 40 3G Gliclazide Modified Release Tablets
- 40 MG Gliclazide Tablets IP
- 60 MG Gliclazide Modified Release Tablets
- Gliclazide And Metformin Hydrochloride Tablets
- Pharmaceutical Glimeporode Tablets IP
- Glimeporode Tablets IP
- Acetazolamide IP 250 mg TAB
- Acarbose IP 25 mg Tab
- Acarbose IP 50 mg tab
- Dapagliflozin 10 mg tab
- Dapagliflozin 5 mg tab
- Dapagliflozin 10mg + Metformin Hydrochloride IP 1000mg (As sustained release form) tab
- Dapagliflozin 10mg, Glimepiride IP 1mg, Metformin Hydrochloride IP 1000mg tab
- Dapagliflozin 10mg, Glimepiride IP 2mg, Metformin Hydrochloride IP 1000mg tab
- Dapagliflozin 5 mg Teneligliptin Hydrobromide 20 mg , tab
- Dapagliflozin 10 mg Metformin Hydrochloride IP 500 mg (Extended Release) tab
- Dapagliflozin 5 mg Metformin Hydrochloride IP 1000 mg (Extended Release) tab
- Epalrestat IP 50mg tab
- Gliclazide Modified Release 30 mg tab
- Gliclazide IP 40 mg TAB
- Gliclazide IP 60 mg Modified Release Tablet
- Gliclazide IP 80 mg TAB
- Gliclazide (Modified Release ) IP 60 mg, metformin hydrochloride IP 500mg Tablet
- Gliclazide IP 80 mg Metformin Hydrochloride IP 500 mg tablet
- Glimepiride IP 1 mg tablet
- Glimepiride IP 2 mg tab
- Glimepiride IP 3 mg tab
- Glimpiride IP 4 mg tab
- Glimepiride IP 0.5 mg + Metformin Hydrochloride IP 500 mg (In Sustained Release Form ) tab
- Glimepiride IP 1 mg. Metformin Hydrochloride 500 mg TAB
- Glimepiride IP 1 mg Metformin Hydrochloride IP 500 mg (In Sustained Release Form) TAB
- Metformin Hydrochloride IP 500 mg Glimepiride IP 2 mg TAB
- Glimepiride IP 2 mg Metformin Hydrochloride IP 1000 mg (In Sustained Release Form) TAB
- Glimepiride IP 2 mg Metformin Hydrochloride IP 500 mg (In Sustained Release Form) TAB
- Glimepiride IP 3 mg Metformin Hydrochloride IP 1000 mg (In Sustained Release Form) TAB
- Glimepiride IP 3 mg Metformin Hydrochloride IP 500 mg (In Sustained Release Form) TAB
- Glimepiride IP 3 mg Metformin Hydrochloride IP 1000 mg (In Sustained Release Form) TAB
- Glimepiride IP 1 mg, Pioglitazone 15 mg Metformin Hydrochloride IP 500 mg (In Extended Release Form)
- Glimepiride IP 2 mg Pioglitazone 15 mg Metformin Hydrochloride IP 500 mg (In Extended Release Form) TAB
- Linagliptin 5 mg TAB
- Linagliptin...5mg, Dapagliflozin 10mg TAB
- Linagliptin 2.5mg + Metfromin Hydrochloride IP 500mg TAB
- Nateglinide 60 mg, TAB
- Nateglinide 60 mg Metformin HCL IP 500 mg (as sustained release), tab
- Linagliptin 2.5mg + Metfromin Hydrochloride IP 1000mg tab
- Pioglitazone Hydrochloride 15 mg tab
- Pioglitazone Hydrochloride 30mg tab
- Pioglitazone Hydrochloride 7.5 mg Tab
- Pioglitazone 15mg Metformin Hydrochloride IP 500mg tab
- Repaglinide IP 0.5mg tab
- Repaglinide IP 1mg tab
- Saxagliptin hydrochloride 5mg tab
- Sitagliptin Phosphate Monohydrate IP 100 mg tab
- Sitagliptin Phosphate Monohydrate IP 25 mg tab
- Sitagliptin Phosphate Monohydrate IP 50 mg tab
- Sitagliptin Phosphate Monohydrate IP 100 mg , Dapagliflozin 10 mg, tab
- Dapagliflozin 10 mg Sitagliptin phosphate monohydrate, Sitagliptin 100 mg Metformin HCL 500 mg (As Extended release ) tab
- Sitagliptin Metformin HCL IP 1000 mg tab
- Sitagliptin Metformin HCL IP 500 mg, tab
- Dapagliflozin 10mg +Sitagliptin Phosphate Monohydrate IP, Sitagliptin 100mg +Metformin Hydrochloride IP 1000mg (In extended relese form) tab
- Metformin Hydrochloride IP 500 mg tab
- Metformin Hydrochloride IP 850 mg tab
- Metformin Hydrochloride IP 1000 mg tab
- Metformin Hydrochloride IP 500 mg (sustained release tablet )
- Metformin Hydrochloride IP 500 mg( sustained release tablet ) tab
- Teneligliptin Hydrobromide 20 mg
- Teneligliptin 20 mg Metformin Hydrochloride IP 500 mg (In Extended Release Form) TAB
- Teneligliptin Hydrobromide Hydrate , Teneligliptin 20 mg Metformin Hydrochloride IP 1000 mg (As Sustained Release) TAB
- Vildagliptin 50 mg TAB
- Dapagliflozin 10 mg Vildagliptin IP 100 mg (As Sustained release ) Tab
- Dapagliflozin 5 mg Vildagliptin IP 100 mg (As Sustained release ) Tab
- Vildagliptin IP 50mg dapaglifiozin 5mg Propanediol monohydrate tab
- Vildagliptin 50 mg Metformin Hydrochloride IP 500 mg tab
- Vildagliptin 50 mg Metformin Hydrochloride IP 1000 mg tab
- Voglibose IP 0.2 mg tab
- Voglibose IP 0.3 mg
- Voglibose IP 0.2 mg Metformin HCI IP 500 mg
- Voglibose IP 0.3 mg Metformin Hydrochloride IP 500 mg (In extended release form) tab
- Voglibose IP 0.2 mg Glimepiride IP 2 mg Metformin Hydrochloride IP 500 mg (In extended release form)
- Metformin Hydrochloride IP 500 mg.(Extended Release Form) Glimepiride IP 2 mg. Voglibose IP 0.3 mg.
- Respiratory Medicine
- Acethama-FM Acebrophylline Motelukast And Fexofenadine HCl Tablets
- Acethama-M Acebrophylline And Motelukast Tablets
- Acebrophylline Capsules
- Acebrophylline Sustained Release Tablets
- Acebrophylline Motelukast And Desloratadine Tablets
- Etofylline And Theophylline Injection
- Budesonide Formoterol Fumarate REspirator Suspension
- Formoterol Fumarate And Budesonide Powder For Inhalation IP
- Levosalbutamol Hydrochloride And Budesonide Inhalation Suspension
- 100 MG Clotrimazole Pessaries Tablets IP
- Acebrophylline 100 mg
- Acebrophylline 200 mg Sustained Release Tablet
- Acebrophylline 200 mg (In Sustained Release Form) Montelukast Sodium IP Eq.to Montelukast 10 mg Desloratadine 5 mg
- Acebrophylline 200 mg (In Sustained Release Form) Montelukast Sodium IP Eq.to Montelukast 10 mg Fexofenadine Hydrochloride IP 120 mg
- Etofylline I.P. 84.7mg Theophylline anhydrous (eq. to Theophylline I.P. Hydrate) I.P. 25.3mg water injection I.P.
- Acebrophylline 200 mg (In Sustained Release Form) Montelukast Sodium IP Eq.to Montelukast 10 mg tab
- Acetylcysteine B.P. 20% w/v water for injction
- N-Acetylcysteine BP 600 mg TAB
- Acebrophylline 100 mg N-Acetylcysteine BP 600 mg
- N-Acetylcysteine USP 150 mg Taurine USP 500 mg
- Acetylcysteine B.P. 20% w/v water for injction
- Aminophylline I.P, Anhydrous Aminophylline 25mg Water for injection I
- Salmeterol 50 mcg Fluticasone Propionate IP 500 mcg, (Rota capsule)
- Salmeterol 50 mcg Fluticasone Propionate IP 250 mcg ( Rota capsule)
- Bromhexine Hydrochloride 100ML SYRUP
- Budesonide IP 0.5mg +Formaterol Fmarate Dihydrate 20mcg RESPULES
- Budesonide IP 1mg Formoterol Fumarate Dihydrate 20MCG RESPULES
- Levosalbutamol Hydrochloride IP Eq. to Levosalbutamol 1.25mg Budesonide IP 0.5mg RESPULE
- Respules contains Budesonide IP 0.5mg
- Respules contains Budesonide IP 1mg
- Rotacapsule and gelatin capsule contains Formoterol Fumarate Dihydrate IP Eq. to Formoterol Fumarate 6 mcg Budesonide IP 200 mcg,
- Formoterol Fumarate Dihydrate IP Eq. to Formoterol Fumarate 6 mcg Budesonide IP 400 mcg, ROTA CAPSULE
- Deflazacort 6mg Flavoured syrup
- Cycloserine IP 250 mg hard gelatin capsule
- Dexamethasonate IP 8 mg TABLET
- Dexamethasonate IP 4 mg TABLET
- Doxofylline IP 400 mg TAB
- Doxofylline IP 400 mg Acebrophylline 100 mg TAB
- Doxofylline IP 400 mg Montelukast Sodium , Montelukast 5 mg TAB
- Indacaterol maleate maleate 110mcg., Glycopyrronium Bromide 50mcg Capsule
- Glycopyrronium 25mcg In an Isotonic Solution Respule
- Hydrocortisone IP 10 mg
- Hydrocortisone IP 20 mg TAB
- Ipratropium Bromide,500mcg. Isotonic Solution respules
- Isoniazid IP 100 mg tab
- Isoniazid IP 300 mg tab
- Ipratropium Bromide IP Eq. to Ipratropium Bromide (Anhydrous) 500mcg + Levosalbutamol Sulphate IP Eq. to Levosalbutamol 0.63mg + in an Isotonic Solution 2.5ml Respules
- Levosalbutamol Sulphate , Levosalbutamol 1.25 mg Ipratropium Bromide , Ipratropium Bromide (Anhydrous) 500 mcg In An Isotonic Solution . 2.5ml Respules
- Levosulbutamol Sulphate 0.31mg 2.5ml. Respules
- Levosalbutamol Sulphate 0.63mg ,Isotonic Solution 2.5ml contains
- Levosalbutamol Sulphate 1.25mg Isotonic Solution 2.5ml Respules
- 4ml ampoule contains Sodium chloride IP 3% w/v Water for injection
- Hard Gelatin Capsule Contains Oseltamivir Phosphate 75 mg TAB
- Pyrazinamide IP 500MG tablet
- Pyrazinamide IP 750MG TAB
- Salbutamol Sulphate , Salbutamol 2.5mg. Isotonic Solution RESPULES
- capsule contains Tiotropium Bromide Monohydrate IP eq. to Tiotropium 18 mcg. rota capsule Rotacapsule.
- Ciclesonide IP 400mcg +Formoterol fumarate Dihydrate IP 12mcg +Tiotropium bromide IP 18mcg CAPSULE
- formoterol Fumarate 6mcg + Budesonide IP 200mcg
- formoterol Fumarate 6mcg + Budesonide IP 400mcg inhaler
- Cardic Care Medicine
- 10 MG Amlodipine Tablets IP
- 2.5 MG Amlodipine Tablets IP
- Amlodipine Besylate And Atenolol Tablets
- 2.5 MG ApixabanTablets
- 5 MG ApixabanTablets
- Atropine Sulphate Injection IP
- 40 MG Azilsartan Medoxomil Tablets
- 4 MG Benidipine Hydrochloride Tablets
- 8 MG Benidipine Hydrochloride Tablets
- 5 MG Bisoprolol Fumarate Tablets USP
- Amiodarone HCL IP 100mg, TAB
- Amiodarone Hydrochloride IP 200 mg TAB
- Amlodipine Besylate 10 mg TAB
- Amlodipine Besylate 2.5 mg TAB
- Amlodipine Besylate 5mg
- Amlodipine Besylate 5MG, Atenolol IP 50 mg TAB
- Apixaban 2.5 mg TAB
- Apixaban 5 mg TAB
- Atropine Sulphate 1mg Injection
- Azilsartan Medoxomil 40 mg tablet
- Benidipine Hydrochloride 4 mg tab
- Benidipine Hydrochloride 8 mg
- Bisoprolol Fumarate USP 2.5 mg
- Bisoprolol Fumarate USP 5 mg
- Bisoprolol Fumarate IP 5 mg, Amlodipine Besylate IP 2.5mg, tab
- Bosentan Monohydrate 125mg tab
- Undenatured Type II Collagen, Cholecalciferol Calcium Carbonate, Vitamin C & Mineral Soft Gelatin Capsule.
- Bosentan Monohydrate 62.5 mg TAB
- Calcium Acetate USP-667 mg
- Ivabradine Hydrochloride 7.5MG
- Sodium Picosulfate BP 10 mg
- Ivabradine Hydrochloride 5MG
- Chlorthalidone IP 6.25 mg
- Chlorthalidone IP 12.5 mg
- Cilostazol IP 100 mg TAB
- Cilostazol IP 50 mg TAB
- Cilnidipine IP 10 mg TAB
- Cilnidipine IP 20 mg
- Cilnidipine IP 5 mg TAB
- Clonidine Hydrochloride IP 100 mcg
- Clopidogrel Bisulphate 75MG
- Atorvastatin calcium 10MG, Titanium Dioxide, Clopogril 75mg Capsule
- Clopidogrel Bisulphate 75 mg, Aspirin IP 150 mg tab
- Clopidogrel 75 mg. Aspirin IP 75 mg tab
- Atorvastatin Calcium IP Equivalent to Atorvastatin 20mg (As Pellets) +Clopidogrel Bisulphate IP Equivalent to Clopidogrel (As Pellets) Capsule
- Carvedilol IP 12.5 mg
- Carvedilol IP 3.125 mg
- Carvedilol IP 6.25 mg
- Carvedilol IP 10 mg
- Digoxin IP 0.25 mg
- Diltiazem Hydrochloride IP 30 mg, Sustained Release tablet
- Diltiazem Hydrochloride IP 60 mg Diltiazem Hydrochloride IP 60 mg
- Diltiazem Hydrochloride IP 90 mg sustained release tablet
- Enalapril Maleate IP 2.5 mg
- Enalapril Maleate IP 5 mg
- Eplerenone IP 25mg,
- S(-)Amlodipine Besylate 2.5 mg
- Ezetimibe IP 10 mg
- Fenofibrate IP 145 mg
- Fenofibrate IP 160 mg
- Fenofibrate IP 200 mg
- Fluvastatin Sodium 40 MG
- Frusemide I.P. 20 mg Water for injection
- Furosemide IP 40 mg
- Heparin sodium IP 5000IU (25000IU/5ml) benzyl alcohol 0.95 % v/v Injection
- Heparin sodium IP 1000IU (5000IU/5ml) benzyl alcohol 0.95 % v/v injection
- Hydrochlorothiazide IP 12.5 mg
- Hydrochlorothiazide IP 25 mg
- Isosorbide Mononitrate 10 mg
- Isosorbide Dinitrate 20 mg
- Potassium Chloride BP 1.5g. Inection
- Labetalol Hydrochloride IP 100 mg TAB
- Lisinopril , Aanhydrous Lisinopril 10 mg
- Lisinopril , Aanhydrous Lisinopril 2.5 mg
- Lisinopril Aanhydrous Lisinopril 5 mg
- Losartan Potassium IP 25 mg
- Losartan Potassium IP 50 mg
- Losartan Potassium 50 mg Amlodipine Besylate 5 mg
- Losartan Potassium IP 50 mg Hydrochloride IP 12.5 mg
- Midodrine HCL USP 2.5 mg,
- Midodrine Hydrochloride USP 5mg
- Metoprolol Tartrate IP 25 mg
- Metoprolol Tartrate IP 50 mg
- Metoprolol Succinate 50 mg (As prolonged -Release Form) Amlodipine Besylate 5 mg
- Metoprolol Succinate IP 95 mg , Metoprolol Tartrate 100 mg
- Metoprolol Succinate IP 11.875 mg , Metoprolol Tartrate 12.5 mg
- Metoprolol Succinate IP 23.75 mg , Metoprolol Tartrate 25 mg
- Metoprolol Succinate IP 47.5 mg , Metoprolol Tartrate 50 mg
- Moxonidine BP 0.2 mg
- Moxonidine BP 0.3 mg
- Nicorandil IP 10 mg
- Nicorandil IP 5 mg
- Nebivolol Hydrochloride IP 2.5 mg
- Nebivolol Hydrochloride IP 5 mg
- Nebivolol Hydrochloride IP 5 mg Amlodipine Besylate IP 5 mg
- Nifedipine IP 10 mg Extended Release Tablet
- Nifedipine IP 20 mg Extended Release Tablet
- Nicoumalone IP 1 mg
- Nicoumalone IP 2 mg
- Noradrenaline 1mg water for injection
- Olmesartan Medoxomil 20 mg. Hydrochlorothiazide IP 12.5 mg
- Olmesartan Medoxomil IP 20 mg
- Olmesartan Medoxomil 10 mg
- Nimodipine BP 30 mg
- Nicoumalone IP 4 mg
- Nicoumalone IP 3 mg
- Olmesartan Medoxomil IP 40 mg. Hydrochlorothiazide IP 12.5 mg
- Olmesartan Medoxomil IP 40 mg Amlodipine Besylate IP Eq. to Amlodipine 5 mg Hydrochlorothiazide IP 12.5 mg
- Olmesartan Medoxomil 40 mg
- Perindopril Erbumine BP 4 mg
- Prazosin Hydrochloride IP 5 mg
- Pirfenidone IP 200 mg
- Prazosin Hydrochloride IP Prazosin 2.5 mg
- Prasugrel Hydrochloride 10 mg
- Prasugrel Hydrochloride 5 mg
- Propranolol Hydrochloride IP 40 mg
- Perindopril Erbumine BP 8 mg
- Metoprolol Succinate IP 47.50 mg +. to Metoprolol Tartrate 50mg (As Extended Release) + Olmesartan Medoxomil IP 40mg.
- alcium Dobesilate Monohydrate IP 500 mg
- Olmesartan Medoxomil IP 20 mg Chlorthalidone IP 12.5 mg
- Propranolol Hydrochloride IP 10 mg
- Olmesartan Medoximil IP 40mg + Chlorthalidone IP 12.5mg
- Olmesartan Medoxomil IP 40 mg Amlodipine Besylate IP 5 mg
- Ramipril IP 2.5 mg
- Ramipril IP 10 mg
- Rivaroxaban Micronised 15 mg
- Rivaroxaban Micronized 2.5mg.
- Rosuvastatin Calcium 10mg
- Rosuvastatin Calcium 20 mg
- Rosuvastatin Calcium IP 40 mg
- Rosuvastatin Calcium IP 10 mg (As Granules) Aspirin IP 75 mg (As gastro-resistant tablet)
- Rosuvastation Calcium IP 20 mg (As Granules) Aspirin IP 75 mg (As enteric coated tablet)
- Rosuvastatin Calcium IP Eq.to Rosuvastatin 10 mg (As pellets) Clopidogrel Bisulphate IP 75 mg (as pellets)
- Rosuvastatin Calcium IP Eq.to Rosuvastatin 20 mg (As pellets) Clopidogrel Bisulphate IP mg (As pellets)
- Rosuvastatin Calcium IP Eq. to Rosuvastatin 10 mg Fenofibrate IP 160 mg
- Rosuvastatin Calcium IP Eq.to Rosuvastatin 10 mg (As Granules) Clopidogrel Bisulphate IP Eq.to Clopidogrel 75 mg (As Granules) Aspirin IP 75 mg (As Gastro-resistant tablets IP)
- Rosuvastatin Calcium IP Eq. to Rosuvastatin 20 mg (As Film Coated Tablet) Aspirin IP 75 mg (As Gastro-resistant tablets IP) Clopidogrel Bisulphate IP Eq.to Clopidogrel 75 mg (As Granules)
- Ranolazine 500 mg Sustained Release Tablet
- Atenolol IP 25 mg
- Atenolol IP 50 mg
- Cinnarizine IP 25 mg
- Spironolactone IP 25 mg
- Spironolactone IP 50 mg
- Spironolactone IP 50 mg, Frusemide IP 20 mg
- Tamsulosin Hdyrochloride IP 0.4 mg (As Modified Release)
- Tamsulosin HCl IP 0.4 mg Dutasteride 0.5 mg
- Tamsulosin Hydrochloride IP 0.4 mg Finasteride IP 5 mg
- Telmisartan IP 40 mg Chlorthalidone IP 6.25 mg
- Telmisartan IP 20 mg
- Telmisartan IP 40 mg
- Telmisartan IP 80 mg
- Telmisartan I.P. 80mg, Hydrochlorothiazide I.P. 12.5mg.
- Telmisartan IP 40 mg Hydrochlorothiazide IP 12.5 mg Amlodipine Besylate IP 5 mg
- Telmisartan IP 40 mg Amlodipine Besylate IP 5 mg
- Azelnidipine IP 8 mg Telmisartan IP 40mg,
- Telmisartan 40mg +Bisoprolol 2.5mg.
- Telmisartan IP 40 mg Cilnidipine 10 mg
- Telmisartan IP 40 mg Chlorthalidone IP 12.5 mg Cilnidipine 10 mg
- Telmisartan IP 40 mg Chlorthalidone IP 12.5 mg
- Telmisartan IP 40 mg Hydrochlorothiazide IP 12.5 mg
- Telmisartan IP 40 mg (In Immediate Release Form) Metoprolol Succinate IP 23.75 mg Eq. to Metoprolol Tartrate 25 mg (In Extended Release Form)
- Telmisartan IP 40 mg (In Immediate Release Form) Metoprolol Succinate IP 47.50 mg Eq. to Metoprolol Tartrate 50 mg (In Extended Release Form)
- Nebivolol Hydrochloride IP Eq. to Nebivolol 5mg + Telmisartan IP 40mg
- Telmisartan IP 40mg +Rosuvastatin Calcium IP Eq. to Rosuvastatin 10mg
- Ticagrelor IP 60 mg
- Ticagrelor IP 90 mg
- Tolvaptan IP 15 mg
- Tolvaptan IP 30 mg
- Torsemide IP 10 mg
- Torsemide IP 100 mg
- Torsemide IP 20 mg
- Torsemide IP 40 mg
- Torsemide IP 5 mg
- Torsemide IP 10 mg Spironolactone IP 50 mg
- Torsemide IP 10mg. + Spironolactone IP 25mg.
- Trimetazidine Dihydrochloride IP 35 mg Modified Release Tablet
- Trimetazidine Dihydrochloride IP 60 mg , Modified Release Tablet
- Valsartan IP 40 mg
- Valsartan IP 80 mg
- Valsartan IP 160mg
- Sacubitril 49 mg + Valsartan 51 mg
- Sacubitril Sodium , equivalent to Sacubitril 97mg valsartan IP 103mg
- Sacubitril 24 mg + Valsartan 26 mg
- Valsartan IP 80 mg +Amlodipine Besylate IP Eq. to Amlodipine 5mg
- Atorvastatin 10 mg
- Atovastatin 20 mg
- Atovastatin 40 mg
- Atorvastatin Calcium IP 80 mg
- Atorvastatin Calcium IP Eq.to Atorvastatin 10 mg (As Granules) Aspirin IP 75 mg (As Gastro Resistant Tablet)
- Atorvastatin Calcium IP Eq.to Atorvastatin 20 mg (As Granules) Aspirin IP 75 mg (As Gastro Resistant Tablet)
- Atorvastatin Calcium IP 10 mg Ezetimible IP 10 mg
- Atorvastatin Calcium IP Eq. to Atorvastatin 10 mg Fenofibrate IP 160 mg
- Atorvastatin Calcium IP Eq. to Atorvastatin 10 MG (As Granules)Clopidogrel Bisulphate IP Eq.to Clopidogrel 75 mg (As Granules) Aspirin Ip 75 mg (As Gastro Resistant Tablet)
- Atorvastatin 20mg. (As Granules) + Clopidogrel Bisulphate IP Eq. to Clopidogrel 75mg. (As Granules) + Aspirin IP 75mg. (As Gastro Resistant Tablet)
- Warfarin Sodium Clathrate IP (Anhydrous) 2 mg
- Warfarin Sodium Clathrate IP Eq.to (Anhydrous) 5 mg
- Torsemide IP 10mg. + Spironolactone IP 25mg
- Ayurvedic Products
- Neurologic Medicine
- 100 MG Amisulpride Tablets IP
- 200 MG Amisulpride Tablets
- 10 MG AmitriptylineHydrochloride Tablets IP
- 25 MG AmitriptylineHydrochloride Tablets IP
- 50 MG Brivaracetam Tablets
- 10 MG Aripipeazole Tablets IP
- Sodium Valproate And Valproic Acid Extended Release Tabltes
- Sodium Valproate And Valproic Acid Controlled Release Tabltes
- 200 MG Sodium Valproate Tabltes IP
- 100 MG Allopurinol Tablets IP
- 300 MG Allopueinol Tablets IP
- Ceftazidime IP (Sterile) 1000 mg injection
- Piracetam IP 400 mg TAB
- Piracetam IP 800 mg TAB
- Acamprosate Calcium 333 mg
- Amisulpride IP 100 mg
- Amisulpride IP 200 mg
- Amitriptyline Hydrochloride IP 10 mg
- Amitriptyline Hydrochloride IP 25 mg
- Aripiprazole IP 10 mg
- Sodium Valproate IP 200 mg Valproic Acid IP 87 mg (Both together Correspond Sodium Valproate 300 mg) Extended Release tablet
- Sodium Valproate IP 333 mg Valproic Acid IP 145 mg (Both together Correspond to Sodium Valproate 500 mg) Controlled-Release tablet
- Sodium Valproate IP 200 mg
- Allopurinol IP 100 mg
- Allopurinol IP 300 mg
- Brivaracetam 100 mg.
- Brivaracetam 50 mg
- Blonanserin 4 mg,
- Blonanserin 8 mg,
- Buspirone HCL IP eq. to Buspirone 10mg,
- Buspirone HCL IP eq. to Buspirone 5 mg
- Ceftazidime Pentahydrate I.P Anhydrous Ceftazidime 2000mg. Sterlie Avibactum Sodium 500mg injection
- Citicoline Sodium IP eq. to Citicoline 250 mg. Water for injection IP .
- Citicoline Sodium IP Eqv. To Citicoline 500 mg Piracetam IP 800 mg
- Citicoline Sodium IP 500mg
- Dabigatran Etexilate Mesylate 126.83 mg ,Dabigatran Etexilate 110 mg
- Dabigatran Etexilate Mesylate 172.95 mg Eq. to Dabigatran Etexilate 150 mg
- Desvenlafaxine Succinate Monohydrate Eq. to Desvenlafaxine 50 mg Extended Release Tablet
- Extended Release tablet contains Divalproex Sodium IP Eq.to Valporic Acid 250 mg
- Extended Release tablet contains Divalproex Sodium IP Eq.to Valporic Acid 500 mg
- Donepezil Hydrochloride IP 10 mg
- Donepezil Hydrochloride IP 5 mg
- Duloxetine Hydrochloride IP Eq.to Duloxetine 20 mg
- Duloxetine Hydrochloride USP 20mg
- Duloxetine Hydrochloride IP 30 mg
- Flupentixol HCL BP , Flupentixol 0.5 mg Melitracen HCL , Melitracen 10 mg,
- Fluoxetine Hydrochloride IP 20MG
- Flunarizine Dihydrochloride BP 10 MG
- Flunarizine Dihydrochloride BP , Flunarizine 5 mg
- Fluvoxamine Maleate IP 50 mg,
- Gabapentin IP 100 mg
- Gabapentin IP 300 mg
- Gabapentin IP 100mg Nortriptyline Hydrochloride IP 10 mg,
- Gabapentin IP 400 mg Nortriptyline HCI IP 10 mg
- Nortriptyline Hydrochloride IP, Eq. to Nortriptyline 10mg + Gabapentin IP 200mg
- Nortriptyline Hydrochloride IP eq. to Nortriptyline 10mg + Gabapentin IP 300mg
- Nortriptyline Hydrochloride IP eq. to Nortriptylin 10mg Gabapentin IP 400mg
- Haloperidol IP 5 mg
- Lacosamide 100 mg
- Levosulpiride 25 mg
- Levosulpiride 75 mg
- Levetiracetam IP 1000 mg
- Levetiracetam IP 250 mg
- Levetiracetam IP 500 mg
- Levetiracetam IP 750 mg
- Levetiracetam I.P. 500 mg + Water for Injection I.P.
- levetiracetam IP 100mg Presevatives methylparaben IP 1.35mg & Propylparaben IP 0.15mg SYRUP
- Levosulpride 12.5 mg Water for injection IP q.s.
- Memantine Hydrochloride USP 10 mg
- Memantine Hydrochloride USP 5 mg
- Donepezil Hydrochloride IP 5 mg Memantine Hydrochloride USP 10 mg
- Methylcobalamin IP 500 mcg, Gabapentin IP 300 mg
- Mirtazapine IP 15 mg
- Mirtazapine IP 7.5 mg
- Mitrojapine IP 30mg orally disintegrating tablet
- Naltrexone Hydrochloride IP 50mg
- Naproxen Sodium USP 500 mg Domperidone Maleate I.P 10mg
- S-Adenosyl- L-Methionine Disulfate P -Toluenesulfonate Eq. to S-Adenosyl -L-Methionine 400mg
- Thiamine HCL I.P 100 mg Riboflavin Sodium Phosphate I.P. 5mg Pyridoxine HCL I.P 100 mg Cyanocobalamin I.P 1000 mcg Nicotinamide I.P 100 mg D-Panthenol I.P. 50 mg Benzyl Alcohol I.P. 09% (As Preservative) Water for Injection IP
- Vitamin B Complex with Vitamin B12
- Thiamine Mononitate 2.0 mg Riboflavin 2.0 mg Pyridoxine Hydrochloride 0.5 mg Niacinamide 25 mg Calcium Pantothenate 1.0 mg Vitamin B 12 1.0 mcg
- Nortriptyline Hydrochloride IP 25 mg
- Olanzapine IP 10 mg
- Olanzapine IP 5 mg
- Olanzapine IP 10 mg Fluoxetine HCL IP 20mg
- Opipramol Dihydrochloride 100 mg
- Opipramol Dihydrochloride 50 mg
- Oxcarbazepine IP 150 mg
- Oxcarbazepine IP 300 mg
- Oxcarbazepine IP 450 mg
- Paroxetine Hydrochloride Hemihydrate IP 12.5 mg
- Paroxetine Hydrochloride Hemihydrate IP Anhydrous Paroxetine 25 mg
- Paroxetine hydrochloride IP 25mg
- Pregabalin IP 150mg
- Pregabalin IP 50 mg.
- Pregabalin IP 75 mg
- Pregabalin IP 75MG Duloxetine Hydrochloride IP EQ to Duloxetine 20 MG.
- Pregabalin IP 75 mg Methylcobalamin IP 750 mcg
- Pregabalin IP 75 mg + Methylcobalamin IP 1500 mcg
- Diclofenac Diethylamine Eq. to Diclofenac Sodium IP 5% w/w + Pregaaaaablin IP 8% w/w +Methyl Salicylate IP 10% w/w + Menthol IP 5% w/w + Capsaicin USP 0.035% w/w Gel Base q.s.
- Pregabalin IP 75 mg (As Sustained Release Form) Nortriptyline HCI IP Eq.to Nortriptyline 10 mg Methycobalamin IP 1500 mcg
- Nortriptyline Hydrochloride IP Equivalent to Nortriptyline 10 mg Pregabalin IP 75 mg
- Phenytoin Sodium IP 100 mg
- Prochlorperazine meleate IP 5 mg
- Piracetam IP 400 mg
- Piracetam IP 800 mg
- Piracetam IP 200 mg water for injection IP
- Piracetam I.P. 100 ML SYRUP
- Dispersible Tablet Proxicam IP 20 mg
- Pramipexole Dihydrochloride Monohydrate BP 0.125 mg Doses
- Quetiapine Fumarate IP 100 MG Sustained Release Tablet
- Quetiapine Fumarate IP 200 MG Sustained Release Tablet
- Quetiapine Fumarate IP 300 MG Sustained Release Tablet
- Quetiapine Fumarate IP 50MG
- Quetiapine Fumarate IP 25MG
- Risperidone BP 2 mg
- Risperidone BP 4 mg
- Ropinirole Hydrochloride USP 0.5MG
- Sertraline Hydrochloride IP 25MG
- Sertraline Hydrochloride IP 50 mg
- Sevelamer Carbonate 400 mg
- Sevelamer Carbonate 800 mg
- Sumatriptan Succinate BP 50Mg
- Tetrabenazine 25mg.
- Tofisopam J.P. 100 mg
- Tofisopam J.P. 50 mg
- Topiramate IP 100 mg
- Topiramate IP 25mg
- Topiramate IP 50 mg
- Trazodone HCL IP 25 mg .
- Trazodone HCL IP 50 mg .
- Trifluoperazine Hydrochloride IP 5MG
- Trihexyphenidyl Hydrochloride IP 2 mg
- Trifluoperazine HCL IP 5 mg Trihexyphenidyl HCL IP 2 mg
- Trihexyphenidyl Hydrochloride IP 2 mg Risperidone IP 2 mg
- Venlafaxine HCL B.P 37.5 mg
- Venlafaxine HCL B.P 75 mg prolonged-release tablet
- Diosmin 450mg Hesperidil 50mg
- Betahistine Hydrochloride IP 16 mg
- Betahistine Hydrochloride IP 8 mg
- vortioxetine 10mg
- Anti Allergic Medicine
- Amantadine Hydrochloride IP 100mg.
- 7 ML Azelastine Hydrochloride And Fluticasone Nasal Spray
- Fluticasone Nasal Spray
- 20 MG Bilastine Tablets
- 40 MG Bilastine Tablets
- Bilastine And Montelukast Tablets
- 10 ML Fluticasone Nasal Spray IP
- Dexamethasone Sodium Phosphate Injection IP
- Fexodenadine Hydrochloride And Montelukast Tablets
- 10 MG Hydroxyzine Hydrochloride Tablets IP
- 25 MG Hydroxyzine Hydrochloride Tablets IP
- Dexamethasone Tablets IP
- Adrenaline Bitartrate IP Injection 0.18% w/v
- Lignocaine HCL. I.P Injection 2%
- Lignocaine Adrenaline Injection 10x30 ml
- Valganciclovir Hydrochloride 450mg
- Ofloxacin I.P 50mg + Metronidazole Benzoate I.P 100mg eq. to Metronidazole, syrup 30ml
- Acebrophyline 50mg + Terbutaline Sulphate IP 1.25mg + Guaiphensin IP 50mg
- Aciclovir IP 200mg.
- Dermalogy Medicine
- Acyclovir Ointment
- 10 MG Isotretinoin Soft Gelatin Capsules IP
- Isotretinoin Soft Gelatin Capsules IP
- Betamethasone Sodium Phosphate Tablets IP
- Silver Sulphadiazine And Chlorhexidine Gluconate Cream
- Benzoyl Peroxide And Clindamycin Phosphate Gel BP
- Benzoyl Peroxide And Clindamycin Phosphate Gel BP
- Clotrimazole Cream IP
- Orthopedic Medicine
- Axclopain-P Aceclofenac And Paracetamol Tablets
- Axclopain-SP Aceclofenac Paracetamol And Serratiopeptidase Tablets
- Axclopain-TP Aceclofenac Paracetamol And Thiocolchiocoside Tablets
- 70 MG Alendronate Sodium Tablets USP
- 100 MG Aceclofenac Tablets IP
- Calcium And Vitamin D3 Tablets IP
- Aceclofenac And Paracetamol Tablets
- Aceclofenac And Paracetamol Serratiopeptidase Tablets
- Aceclofenac And Thiocolchicoside Tablets
- 10 MG BaclofenTablets IP
- 25 MG BaclofenTablets IP
- Alfacalcidol IP 0.25 mcg (1a-Hydroxyvitamin D3)
- Diclofenac Diethylamine IP 1.16% w/w (Eq. to Diclofenac Sodium I.P. 1.00% w/w) Linseed Oil BP 3.00% w/w Methyl Salicylate IP 10.00% w/w Menthol IP 0.5% w/w Benzyl Alcohol IP 1.00% w/w (As Preservative) In Gel Base
- Diclofenac Diethylamine IP 1.16% w/w + Linseed Oil BP 3.00% w/w + Methyl Salicylate IP 10.00% w/w + Menthol IP 0.5% w/w + Benzyl Alcohol IP 1.00% w/w
- Aceclofenac IP 100 mg Paracetamol IP 325 mg Cetirizine Dihydrochloride IP 10mg Phenylephrine Hydrochloride IP 5 mg Caffeine ( Anhydrous) IP 25 mg
- Aceclofenac IP 100 mg. Paracetamol IP 325 mg. Chlorzoxazone USP 250 mg.
- Aceclofenac IP 100 mg. Paracetamol IP 325 mg. Chlorzoxazone USP 250 mg.
- Sustained Release tablet contains Aceclofenac I.P 200MG
- Osteoarthritis,Spondylitis,Low Back Pain,Muscular pain OIL
- Calcium Carbonate Ip 1250 mg Eq. to elemental Calcium 500 mg Vitamin D3 Ip 2000 I.U. Methylcobalamin IP 1500 mcg L-Methylfolate Calcium 1mg Pyridoxal 5 Phosphate 0.5 mg
- Dicyclomine HCI IP 20 mg. + Paracetamol IP 325 mg.
- Glucosamine Sulphate Potasium chloride USP 750MG + Methyl Sulphonyl Methane USP 250MG + Diacerein 50MG
- Colchicine IP 0.5 mg
- Deflazacort 1mg
- Deflazacort 30 mg
- Deflazacort 6 mg
- Etoricoxib IP 120 mg
- Etoricoxib IP 60 mg
- Etoricoxib IP 90 mg
- Etoricoxib IP 60 mg Paracetamol IP 325 mg
- Etoricoxib IP 60 mg Thiocolchicoside IP 4 mg
- Flupirtine Maleate 100 mg
- Hyoscine Butylbromide IP 20 mg. water for injection IP
- Hydroxychloroquine Sulphate IP 200 mg
- Ibandronic Acid Monosodium Monohydrate Acid 150 mg
- Indomethacin IP 75 mg
- Ketorolac Tromethamine IP 10 mg
- Ketorolac Tromethamine IP 10 mg
- Leflunomide IP 10 mg
- Leflunomide IP 20 mg
- Linseed Oil BP 3.00% w/w. Diclofenac Diethylamine BP 1.16% w/w. (Equivalent to Diclofenac Sodium 1.00% w/w) Methyl Salicylate IP 10.00% w/w Menthol IP 0.5% w/w Benzyl Alcohol IP (As Preservative) 1.00% w/w/
- Naproxen Sodium 250 mg
- Naproxen Sodium 500 mg
- Nutritional Info : Each Sachet [5g] Powder Contains [Approx] : Energy 5.68kcal + Carbohydrate 1.42g + Sugar 0.30g + Protein 0.00g + Fat 0.00g + Silymarin Extract 1.00g + L-Ornithine L-Aspartate 35.00mg
- Blend of scutellaria baicalensis and acacia catechu
- Polmacoxib 2MG
- Diclofenac Diethylamine IP 1.16% w/w Eq. to Diclofenac Sodium 1.00 % w/w Methylsalicylate IP 10.00% w/w Linseed oil BP 3.00`% w/w Menthol IP 5.00% w/w Excipients & propellant 100 % w/w SPRAY
- Osteoarthritis Spondylitis Low Back Pain Muscular pain PAIN OIL
- Diclofenac Diethylamine IP 2.32% w/w + Eq. to Diclofenac Sodium 2% w/w + Methyl salicylate IP 10.0% w/w + Virgin Linseed oil BP 3.0`% w/w + Menthol IP 5.0% w/w SPRAY
- Serratiopeptidase IP 10 mg
- Diclofenac Potassium BP 50 mg. + Serratiopeptidase IP 10 mg.
- Thiocolchicoside IP 4 mg
- Diclofenac Sodium IP 75 mg. Benzyl Alcohol IP 4% w/v.
- Diclofenac Potassium BP 100 mg sustained release tablet
- Diclofenac Sodium I.P. 75 mg Benzyl Alcohol I.P. 4% v/v Water for Injection I.P.
- Diclofenac Sodium IP 50 mg.
- Diclofenac Sodium I.P 50MG + Paracetamol I.P 325
- Diclofenac Potassium BP 50 mg. Paracetamol IP 325 mg. Serratiopeptidase IP 10 mg.
- Diclofenac Potassium BP 50 mg + Paracetamol IP 325 mg + Serratiopeptidase IP 10
- Diclofenac Diethylamine IP 1.16% w/w (Eq. to Diclofenac Sodium 1%w/w) In a gel base
- Gastroenterologic Medicine
- 100 MG Acotiamide Tablets
- 0.5 MG Entecavir Tablets IP
- 1 MG Entecavir Tablets IP
- 200 ML Cyproheptadine Hydrochloride Tricholine Citrate And Sorbitol Solution
- Psyllium Powder
- ErgotamineCaffeine Paracetamol And Prochlorperazine Maleate Tablets
- 100 ML Lactulose Solution USP
- 10 ML Dicyclomine HCl And Activated Dimethicone Drops
- Bisacodyl Tablets IP
- Loperamide Hydrochloride Capsules IP
- 200 ML Lactulose Solution USP
- Veginal Wash
- Herbal Products
- Contact Us
- Home
- Products
- Anti Diabetic Medicine
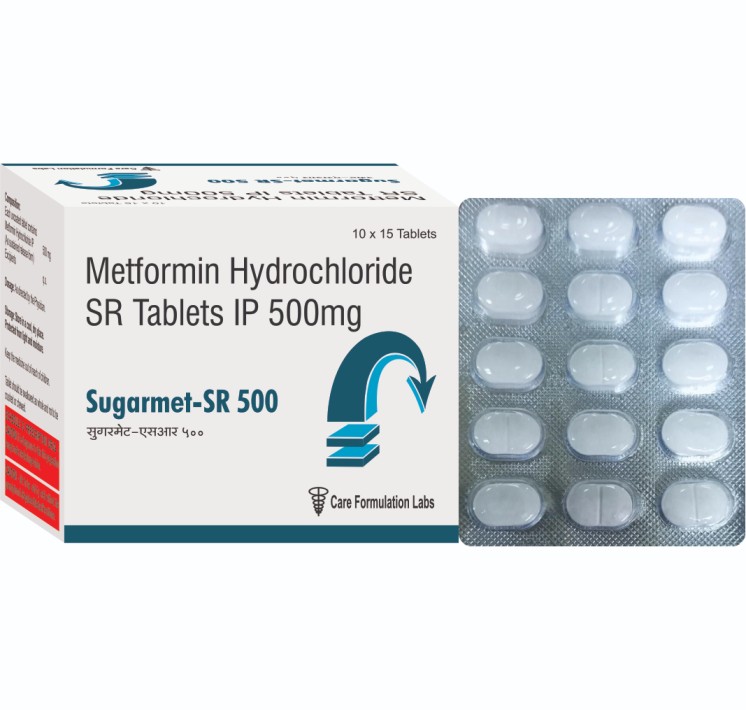
- Metformin Hydrochloride IP 500 mg (sustained release tablet )
Metformin Hydrochloride IP 500 mg (sustained release tablet )
33.1 INR/Strip
Product Details:
- Purity High
- Type Tablets
- Ingredients Other
- Feature Control blood sugar levels
- Shelf Life 12 Months
- Storage Instructions Dry Place
- Click to View more
-
Share Your Product:
X
Metformin Hydrochloride IP 500 mg (sustained release tablet ) Price And Quantity
- 33.1 INR/Strip
- 100 Pack
Metformin Hydrochloride IP 500 mg (sustained release tablet ) Product Specifications
- Tablets
- Dry Place
- 12 Months
- Control blood sugar levels
- High
- Other
Metformin Hydrochloride IP 500 mg (sustained release tablet ) Trade Information
- 5000 Pack Per Month
- 7 Days
Product Description
Description
- Each uncoated sustained release tablet contains: Metformin Hydrochloride IP 500 mg + Excipients q.s.
Doses
- As Directed by Physician
Indication
- Metformin is an oral diabetes medicine that helps control blood sugar levels. Metformin is used together with diet and exercise to improve blood sugar control in adults with type 2 diabetes mellitus. Metformin is sometimes used together with insulin or other medications, but it is not for treating type 1 diabetes.
FAQs of Metformin Hydrochloride IP 500 mg (sustained release tablet ):
Q: What is the shelf life of Metformin Hydrochloride IP 500 mg (sustained release tablet)?
A: The shelf life of Metformin Hydrochloride IP 500 mg (sustained release tablet) is 12 months.Q: What are the main ingredients in Metformin Hydrochloride IP 500 mg (sustained release tablet)?
A: The main ingredient in Metformin Hydrochloride IP 500 mg (sustained release tablet) is Metformin Hydrochloride.Q: What is the purity level of Metformin Hydrochloride IP 500 mg (sustained release tablet)?
A: The purity of Metformin Hydrochloride IP 500 mg (sustained release tablet) is high.Q: What is the main feature of Metformin Hydrochloride IP 500 mg (sustained release tablet)?
A: The main feature of Metformin Hydrochloride IP 500 mg (sustained release tablet) is to control blood sugar levels.Q: How should Metformin Hydrochloride IP 500 mg (sustained release tablet) be stored?
A: Metformin Hydrochloride IP 500 mg (sustained release tablet) should be stored in a dry place.Tell us about your requirement

Price:
Quantity
Select Unit
- 50
- 100
- 200
- 250
- 500
- 1000+
Additional detail
Mobile number
Email
Name
Comapny Name
Phone Number
Email Id
City / State
Confirm Your Requirement
Verification Code
Did not receive yet?
Resend OTP

Youre Done!
We have received your requirements and will reply shortly with the best price.
Products You May Like
Other Products in 'Anti Diabetic Medicine' category
×
Add a mobile number to receive call from "CARE FORMULATION LABS PRIVATE LIMITED"
×
Enter OTP
×
Share additional details for a quick response
×
Thank You!
Thank You for your valuable time. We have received your details and will get back to you shortly.
×
Get a price quote for Metformin Hydrochloride IP 500 mg (sustained release tablet )
×
We accept minimum 10 boxes of all products.
Contact Us
- B No. 673, Block-E -673, DSIIDC, Industrial area, Narela,Delhi - 110040, India
- Phone : +917971671193 PIN:( 207 )
CARE FORMULATION LABS PRIVATE LIMITED
GST : 07AAECC3106E1Z9
- Mr Naveen Kumar Jindal (Director)
- Mobile : 917971671193 PIN:( 207 )
- Send Inquiry
- Direct No. : +919717230298
Our Products
- Herbal Products
- Urology And Nephrology Medicine
- Erectile Dysfunction Medicine
- Pharmaceutical Injection
- Endocrinology Tablet
- Oncology Medicine
- Ophthalmic Medicine
- Gynecologic Medicine
- Pain Relief Medicine
- HIV And Aids Medicine
- General Medicine
- Anti Infective Medicine
- Cosmetic Products
- Anti Diabetic Medicine
- Respiratory Medicine
- Cardic Care Medicine
- Ayurvedic Products
- Neurologic Medicine
- Anti Allergic Medicine
- Dermalogy Medicine
- Orthopedic Medicine
- Gastroenterologic Medicine
- Veginal Wash
CARE FORMULATION LABS PRIVATE LIMITED
All Rights Reserved.(Terms of Use)
Developed and Managed by Infocom Network Private Limited.
Developed and Managed by Infocom Network Private Limited.

 Send Inquiry
Send Inquiry
 English
English Spanish
Spanish French
French German
German Italian
Italian Chinese (Simplified)
Chinese (Simplified) Japanese
Japanese Korean
Korean Arabic
Arabic Portuguese
Portuguese